Bergman cyclization of main-chain enediyne polymers for enhanced DNA cleavage†
Abstract
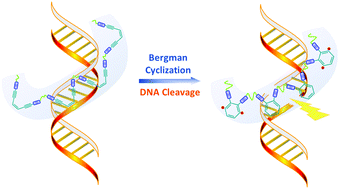
Since the discovery of the role of enediynes in natural antibiotics (such as calicheamicines) via in situ diradical-induced DNA strand cleavage, Bergman cyclization has attracted fervent attention for decades. Thus, Bergman cyclization is widely used in pharmaceutics, synthesis, and polymer chemistry as a trigger for the generation of diradicals. Whereas applications of the Bergman cyclization in polymers mostly rely on side-chain linked enediynes, strategies to embed enediynes as the main repeating units into polymers are not reported yet. Here, the synthesis of main-chain enediyne polymers was accomplished, allowing to embed and control the reactivity of the diamino enediynes via polycondensation into polyimines. Such main-chain enediyne polymers in solution exert a chain-length dependent DNA cleavage activity under physiological conditions, additionally tunable by modulating the stereoelectronic environment via their substitution patterns. Photochemical activation generates long-lived free radicals as verified via electron paramagnetic resonance (EPR) spectroscopy, with rates of their formation comparable to those in DNA cleavage experiments.


 Please wait while we load your content...
Please wait while we load your content...