Sulfur–DMSO promoted oxidative coupling of active methylhetarenes with amines: access to amides†
Abstract
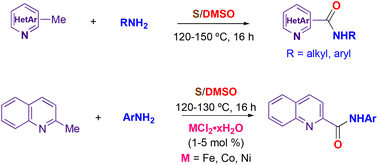
The elemental sulfur–DMSO couple was found to efficiently promote the oxidative coupling of active methylhetarenes with amines to yield amides under simple heating conditions. When 2-methylquinoline was used as the methylhetarene component, the formation of the expected 2-quinolinecarboxamides from anilines could be efficiently catalyzed by iron, nickel and cobalt salts. The method displayed good functional group tolerance and was applicable to aromatic, heteroaromatic and aliphatic amines. Other substrates such as phenylacetic acid, dibenzyl disulfide, and benzylamine could act as competent partners in place of methylhetarenes.


 Please wait while we load your content...
Please wait while we load your content...