Nickel-catalyzed cyanation reaction of aryl/alkenyl halides with alkyl isocyanides†
Abstract
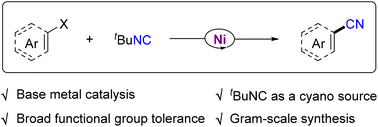
We herein describe a nickel-catalyzed cyanation reaction of aryl/alkenyl halides with alkyl isocyanides. Both aryl/alkenyl iodines and bromides were found to be competent electrophiles that reacted with alkyl isocyanides, affording nitrile compounds in moderate to good yields. A range of functional groups including halogens as well as hydroxyl, formyl, and acetamino groups were fairly compatible with the nickel catalysis. This protocol featured broad functional group tolerance, simple reaction conditions, and gram-scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...