Tandem cyclocondensation of 1,3-bis-sulfonylpropan-2-ones with arylaldehydes. One-pot synthesis of tris-sulfonyl 3-arylphenols†
Abstract
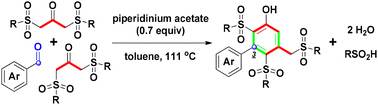
One-pot tandem piperidinium acetate-mediated cyclocondensation of 1,3-bis-sulfonylpropan-2-ones with arylaldehydes generates tris-sulfonyl 3-arylphenols in moderate to good yields in refluxing toluene under easy-operational reaction conditions. A plausible mechanism is proposed and discussed. In the overall reaction process, water and sulfinic acid were generated as the byproducts. Various ammonium carboxylate-promoted conditions are investigated for one-pot [3 + 2 + 1]-benzannulation.


 Please wait while we load your content...
Please wait while we load your content...