AgNTf2 catalyzed cycloaddition of N-acyliminium ions with alkynes for the synthesis of the 3,4-dihydro-1,3-oxazin-2-one skeleton†
Abstract
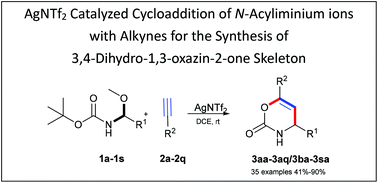
A catalyzed process for the synthesis of the 4,6-substituted 3,4-dihydro-1,3-oxazin-2-one skeleton has been developed through cycloaddition of in situ generated acyliminium intermediates with alkynes. A range of chain N,O-acetals and terminal alkynes were amenable for this mild transformation. As a result, a series of desired cycloaddition products were obtained in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...