Three modes of interactions between anions and phenolic macrocycles: a comparative study†
Abstract
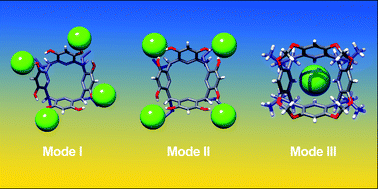
Macrocyclic polyphenolic compounds such as resorcin[4]arenes can be considered as multidentate anion receptors. In the current work, we combine new experimental data and reports from the previous literature (solution data and deposited crystal structures from the CCDC) to systematically analyze binding motifs between resorcin[4]arene derivatives and anions, determine the role of supporting interactions from CH donors, ion pairing and estimate their relative strength. We have found that in medium polarity solvents (THF) anion binding is a main driving force for the formation of complexes between resorcinarenes and Alk4NX salts. Three binding modes have been detected using 1H NMR and DOSY, depending on the type of additional interactions. Mode I was observed for upper-rim unsubstituted resorcinarenes, which use OH groups and aromatic CH from the upper rim as hydrogen bond donors to form multidentate and multivalent binding sites at the upper rim. Mode II was observed for upper-rim halogenated resorcinarenes (tetrabromo- and tetraiodo-derivatives), which use OH groups and aliphatic CH atoms from the bridges to support the chelation of anions between aromatic units. This binding mode is also multidentate and multivalent, but weaker and more anion-selective than mode I (works effectively for chlorides but not for bromides). For O-substituted derivatives, mode III is observed, with anions bound in a nest formed by aromatic CH atoms in the lower rim (multidentate but monovalent binding). The relative strength of these three binding modes, their solvent-dependence, and emergence in the crystal structures (CCDC) have been evaluated.


 Please wait while we load your content...
Please wait while we load your content...