Preparation of glycosyl carboxylic acids via stereoselective synthesis and oxidative cleavage of C-vinyl glycosides†
Abstract
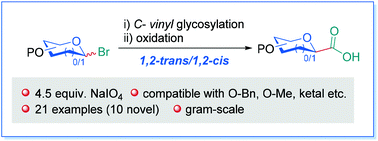
We have developed an improved cyanide-free strategy for the synthesis of glycosyl carboxylic acids, employing stereoselective C-vinyl glycosylation and oxidative cleavage of C-vinyl glycosides as key steps. Compared to our previous work, the amount of NaIO4 required for the oxidative cleavage step is reduced significantly from 18 equivalents to 4.5 equivalents. This modification not only is advantageous in terms of operation and costs but also avoids the over-oxidation problem, thus greatly expanding the substrate scope, which is evidenced by the fact that 10 out of 21 glycosyl carboxylic acids synthesized are undocumented. With differently O5-protected furanosyl acids in hand, we demonstrate that an electron-rich protecting group is beneficial for the decarboxylative arylation of furanosyl carboxylic acids. This represents a rare example of protecting groups affecting the reaction efficiency in radical C-glycosylation. As C-vinyl glycosides can be prepared stereoselectively and the oxidative step is stereoretentive, the approach provides an effective means to access 1,2-trans or 1,2-cis glycosyl acids, which would be a valuable alternative to the cyanide-based synthesis of glycosyl carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...