Theoretical mechanistic insights into the polar hydrohalogenation of olefins†
Abstract
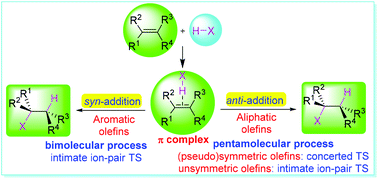
Polar hydrohalogenation of olefins, also called electrophilic addition of hydrogen halides to olefins, is an essential, classical, and important organic reaction. Mechanistic and stereoselective insights into the polar hydrohalogenations of nine structurally different distinct olefins were obtained through theoretical investigation via density functional theory (DFT) calculations. The results indicate that, after the formation of π-complexes of hydrogen halides and olefins, all olefins can undergo bimolecular intimate ion-pair syn-addition processes. However, aliphatic olefins can also undergo a pentamolecular concerted anti-addition process through a cyclic proton transferring mechanism, with a fourteen-membered cyclic transition state composed of olefins, two molecules of hydrogen halides, and an acetic acid dimer for the proton transfer. Although unsymmetric aliphatic olefins exhibit an obvious electrostatic intimate ion-pair process in their anti-addition, the process is still concerted with significantly asynchronous characteristics due to a greater hyperconjugation effect. The activation energies of anti-additions are generally lower than those of the corresponding syn-additions, especially for hydrobromination. The relative reaction rate constants of the anti-additions are always larger than those of the corresponding syn-additions on the basis of dynamics treatment according to transition state theory. Thus, in the hydrohalogenations, aliphatic olefins always give anti-adducts as major products, while aromatic olefins produce syn-adducts as major products. The substituent-controlled mechanisms and stereoselectivities are summarized and rationalized. The current investigation provides comprehensive insights into the mechanism and stereoselectivity of hydrohalogenations of olefins.


 Please wait while we load your content...
Please wait while we load your content...