(±)-Walskiiglucinol A, a pair of rearranged acylphloroglucinol derivative enantiomers from Hypericum przewalskii†
Abstract
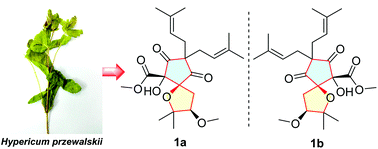
(±)-Walskiiglucinol A (1a/1b), a pair of rearranged acylphloroglucinol derivatives with a new carbon skeleton, was obtained from Hypericum przewalskii. Compounds 1a/1b were the first examples of naturally occurring acylphloroglucinol derivatives possessing a unique 1-oxaspiro[4.4]nonane core bearing a new 5/5 ring system. Their planar and relative structures were identified by extensive spectroscopic analysis and NMR chemical shift calculations with DP4+ probability analysis, and their absolute configurations were determined by electronic circular dichroism (ECD) calculations. A plausible biogenetic pathway of 1a/1b was proposed in which the breakage of the C-2/C-3 linkage via a retro-Claisen reaction and the cyclization between C-3 and C-1 were proposed as key steps. The isolates were evaluated for cytotoxic activities against a panel of cancer cell lines and anti-inflammatory activities against lipopolysaccharide (LPS)-induced NO production, and compounds 1a/1b showed moderate cytotoxic activities with IC50 values ranging from 9.72 to 36.75 μM.


 Please wait while we load your content...
Please wait while we load your content...