Visible light-promoted intermolecular cyclization/aromatization of chalcones and 2-mercaptobenzimidazoles via an EDA complex and a mechanism study†
Abstract
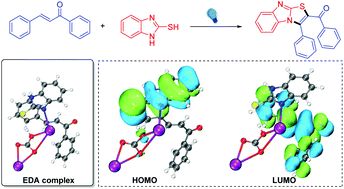
Visible-light-promoted cyclization and aromatization of chalcones with 2-mercaptobenzimidazoles have been successfully developed to obtain diverse imidazo[2,1-b]thiazoles, and C–S and C–N bonds were constructed in one step. The reaction uses oxygen in the air as an oxidant, and the method does not need an external photocatalyst or a transition metal catalyst. The strategy features mild conditions, a simple system, readily accessible feedstocks, and a friendly environment. UV absorption spectroscopy and control experiments have shown that the reaction mechanism involves the formation of an electron-donor–acceptor (EDA) complex from thiolate anions and chalcones. In order to verify the mechanism, we studied the structure and HOMO/LUMO of the EDA complex by density functional theory (DFT) calculations. The results show that the π–π stacking between chalcones and 2-mercaptobenzimidazoles will cause a red shift of the UV absorption wavelength in the presence of Cs2CO3, and also provide a theoretical basis for the electron transfer of EDA complexes.


 Please wait while we load your content...
Please wait while we load your content...