Synthesis of symmetrical secondary oligoethylene glycolated amines from diethanolamine†
Abstract
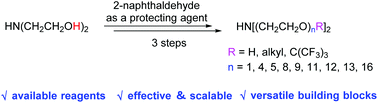
Monodisperse oligoethylene glycols (M-OEGs)-containing symmetrical secondary amines are highly valuable synthetic intermediates in drug development and materials sciences. Scalable three-step synthesis of M-OEGs secondary amines with flexible M-OEGs and/or alkyl chains is described herein. Through reduction amination of diethanolamine, Williamson ether synthesis, and subsequent deprotection, a series of M-OEGs secondary amines with diverse and fine-tunable chemical structures were conveniently prepared. The presented strategy is attractive with readily available starting materials, simple catalytic systems, scalable synthesis, and avoids the use of explosive sodium azide.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...