Efficient synthesis of pentasubstituted pyrroles via intramolecular C-arylation†
Abstract
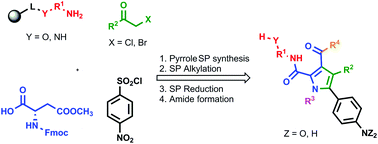
Immobilized L-aspartic acid beta-methyl ester (Fmoc-Asp(OMe)-OH) was reacted with 4-nitrobenzenesulfonyl chloride, followed by alkylation with various α-haloketones. The resulting intermediates were treated with potassium trimethylsilanolate, which yielded tetrasubstituted pyrroles after a one-step transformation consisting of sequential C-arylation, aldol condensation and spontaneous aromatization. The discovered synthetic strategy enables fast and simple access to pentasubstituted and functionalized pyrroles from a number of readily available starting materials.


 Please wait while we load your content...
Please wait while we load your content...