Photoredox-catalyzed fluorodifluoroacetylation of alkenes with FSO2CF2CO2Me and Et3N·3HF†
Abstract
Photoredox-catalyzed three-component fluorodifluoroacetylation of aromatic alkenes is reported, which features a wide substrate scope and functional group tolerance. An advantage of the reaction is the use of a nucleophilic fluoride source and a general difluoroacetylation reagent for the fluorodifluoroacetylation of alkenes.
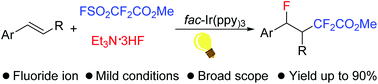


 Please wait while we load your content...
Please wait while we load your content...