Enantioselective synthesis of 2-indolyl methanamine derivatives through disulfonimide-catalyzed Friedel–Crafts C2-alkylation of 3-substituted indoles with imines†
Abstract
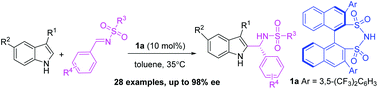
An asymmetric Friedel–Crafts C2-alkylation between 3-substituted indoles and imines catalyzed by chiral BINOL-derived disulfonimides (DSIs) has been developed. This reaction tolerated a wide range of 3-substituted indoles and imines, affording a series of chiral 2-indolyl methanamine derivatives in good yields with good to excellent enantioselectivities (up to 98% ee). This is a useful protocol for the direct synthesis of 2-indolyl methanamine derivatives. It is worth noting that increasing the temperature in this reaction could result in a better enantioselectivity, making it different from the other common organocatalytic systems.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...