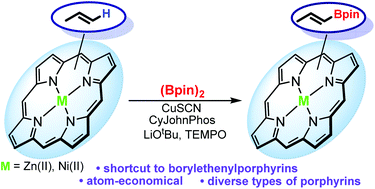
Abstract
A method of direct borylation of vinyl-substituted porphyrinoids (porphyrins and chlorins) has been developed based on the copper catalyzed vinylic C–H activation. Ni(II) complexes of meso- and β-vinylporphyrinoids have been transformed to the corresponding pinacolboronated derivatives with good yields and high (E)-stereoselectivity. The method provides an easy and direct access to the valuable synthons which were shown to act as nucleophylic partners in the Suzuki cross-coupling building tetrapyrrole derivatives with π-conjugation through the carbon–carbon double bond.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...