Studies directed toward the synthesis of hedycoropyrans: total synthesis of des-hydroxy (−)-hedycoropyran B (ent-rhoiptelol B)†
Abstract
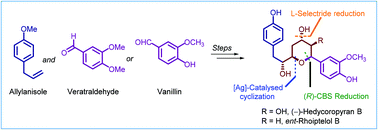
A full account of our efforts directed towards the synthesis of the diarylheptanoid-derived natural products hedycoropyrans that led to the total synthesis of ent-rhoiptelol B is described. In this endeavor, we have attempted two distinct synthetic strategies to access hedycoropyrans A and B, which led us to establish a facile synthetic route for des-hydroxy (−)-hedycoropyran B (ent-rhoiptelol B) from simple and readily accessible building blocks of 4-allylanisole and vanillin, employing Sharpless asymmetric epoxidation, CBS reduction, and an intramolecular AgOTf-catalyzed oxa-Michael reaction of suitably functionalized hydroxy-ynone as key transformations. The investigations disclosed herein will provide insights into designing novel synthetic routes for THP-DAH-derived natural products.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...