Two undescribed pairs of isoprenyl hydroxybenzoic acid derivatives from coculture of Pestalotiopsis sp. and Penicillium bialowiezense: enantioseparation and their absolute configurations†
Abstract
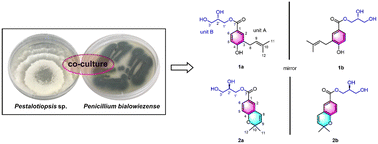
Two undescribed pairs of ester dimer enantiomers, namely glyceryl 4-hydroxy-3-prenyl-benzoate and anofinic glyceride, which represent the first examples of isoprenyl hydroxybenzoic acid derivatives bearing a glycerol moiety, were isolated from coculture of Pestalotiopsis sp. and Penicillium bialowiezense. The separation of enantiomers was achieved via chiral-phase HPLC. Their structures incorporating absolute configurations were characterized based on the extensive spectroscopic data, single-crystal X-ray crystallography analysis, [Mo2(AcO)4]-induced circular dichroism, and comparison of the experimental ECD curves. Additionally, glyceryl 4-hydroxy-3-prenyl-benzoate and anofinic glyceride were evaluated for the β-glucuronidase (GUS) and butyrylcholinesterase (BChE) inhibitory activities, and glyceryl 4-hydroxy-3-prenyl-benzoate showed remarkable GUS inhibitory potency with an IC50 value of 22.31 ± 0.60 μM, which was comparable to the positive control DSA (D-saccharic acid 1,4-lactone monohydrate) (IC50 = 24.30 ± 0.80 μM).


 Please wait while we load your content...
Please wait while we load your content...