Regiospecific route to N9-alkylated thioxanthines†
Abstract
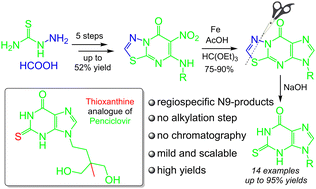
A regiospecific route to N9-alkylated thioxanthines as novel acyclic nucleoside analogues has been developed. This approach is based on a cleavage methodology involving the construction of a target heterocyclic scaffold based on a 1,3,4-2H-thiadiazole moiety, which is subsequently cleaved under basic conditions. The procedure is performed under mild scalable conditions excluding column chromatography purification, with readily available precursors, and results in good to excellent yields of the target thioxanthines along with an analogue of the marketed drug penciclovir.


 Please wait while we load your content...
Please wait while we load your content...