Free-standing Co/Zn sulfide supported on Cu-foam for efficient overall water splitting†
Abstract
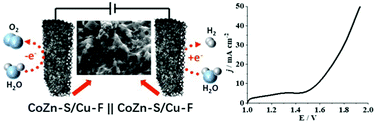
High-performance bifunctional electrocatalysts for both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) are arousing great interest aiming at efficient electrochemical splitting of water. Herein, we report a facile strategy to fabricate Co/Zn sulfide supported on Cu-foam (CoZn-S/Cu-F) as a free-standing electrode with high-performance for overall water splitting. In half-cell electrochemical systems, the overpotentials required to reach the current densities of ±10 mA cm−2 for the OER and HER are only 170 and 130 mV, respectively. After a long-term stability test running 20 000 cyclic voltammograms, the respective potential losses for the OER and HER are only 82 and 41 mV at ±50 mA cm−2. The constructed overall water splitting system driven by simulated solar energy also exhibits prominent performance with gas bubbles continuously generated at both electrodes. A cell voltage of only 1.58 V is required to reach the current densities of ±10 mA cm−2, surpassing the commercialized noble metal and state-of-the-art non-noble metal electrodes. The excellent performance for the overall water splitting is ascribed to the CoS nanoparticles covered with ZnS nanoflocs on the highly conductive Cu-foam, which allows close interfacial contact with the electrolyte, promotes mass and charge transfer, and facilitates desorption of the generated gas from the catalyst surface.


 Please wait while we load your content...
Please wait while we load your content...