Synthesis, cytotoxicity and antioxidant activity of new 1,3-dimethyl-8-(chromon-3-yl)-xanthine derivatives containing 2,6-di-tert-butylphenol fragments†
Abstract
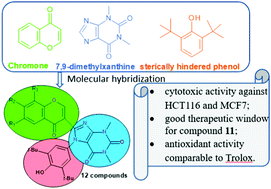
The condensation of 3-formylchromones and 6-amino-5-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)amino)-1,3-dimethyl-pyrimidine-2,4(1H,3H)-dione allowed us to synthesize chromone based hybrids containing additional pharmacophores – xanthine and sterically hindered phenol fragments. The novel compounds have been characterized using 1H and 13C NMR spectroscopy, mass spectrometry and elemental analysis. The molecular structure of compound 2 was determined using single crystal X-ray diffraction studies. Compounds 2–9, 11, and 12 exhibit cytotoxic activity against HCT116 human colon carcinoma cells and MCF7 breast carcinoma cells at submicromolar concentrations. Compound 11 containing a 6,8-dimethylchromone core has the highest cytotoxicity against tumor cells and the lowest against non-tumor fibroblasts – this is the leading compound of the obtained series with acceptable in vitro selectivity. The synthesized compounds exhibit antioxidant activities comparable to the reference drug Trolox. The combination of promising antioxidant activity and selective antitumor cytotoxicity in vitro indicates the promising prospects of these compounds as chemotherapy agents.


 Please wait while we load your content...
Please wait while we load your content...