Molecular dynamics study of the internalization of cell-penetrating peptides containing unnatural amino acids across membranes†
Abstract
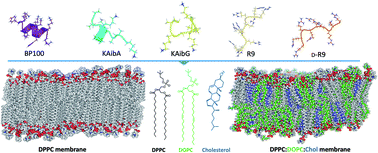
Peptide-based delivery systems that deliver target molecules into cells have been gaining traction. These systems need cell-penetrating peptides (CPPs), which have the remarkable ability to penetrate into biological membranes and help internalize different cargoes into cells through the cell membranes. The molecular internalization mechanism and structure–function relationships of CPPs are not clear, although the incorporation of nonproteinogenic amino acids such as α-aminoisobutyric acid (Aib) has been reported to increase their helicity, biostability and penetration efficiencies. Here, we used molecular dynamics to study two Aib-containing CPPs, poly(LysAibAla)3 (KAibA) and poly(LysAibGly)3 (KAibG), that previously showed high cell internalization efficiency. KAibA and KAibG displayed the lowest internalization energies among the studied CPPs, showing distinct internalization mechanisms depending on the lipid composition of the model membranes. The presence of Aib residues allows these CPPs to adopt amphipathic folding to efficiently penetrate through the membranes. Elucidating how Aib incorporation affects CPP–membrane binding and interactions is beneficial for the design of CPPs for efficient intracellular delivery.


 Please wait while we load your content...
Please wait while we load your content...