Novel etodolac derivatives as eukaryotic elongation factor 2 kinase (eEF2K) inhibitors for targeted cancer therapy†
Abstract
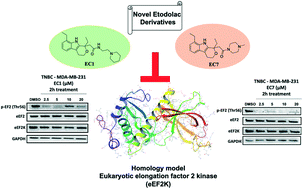
Eukaryotic elongation factor 2 kinase (eEF2K) has been shown to be an important molecular driver of tumorigenesis and validated as a potential novel molecular target in various solid cancers including triple negative breast cancer (TNBC). Therefore, there has been significant interest in identifying novel inhibitors of eEF2K for the development of targeted therapeutics and clinical translation. Herein, we investigated the effects of indole ring containing derivatives of etodolac, a nonsteroidal anti-inflammatory (NSAID) drug, as potential eEF2K inhibitors and we designed and synthesized seven novel compounds with a pyrano[3,4-b] indole core structure. We evaluated the eEF2K inhibitory activity of seven of these novel compounds using in silico molecular modeling and in vitro studies in TNBC cell lines. We identified two novel compounds (EC1 and EC7) with significant in vitro activity in inhibiting eEF2K in TNBC cells. In conclusion, our studies indicate that pyrano[3,4-b] indole scaffold containing compounds demonstrate marked eEF2K inhibitory activity and they may be used as eEF2K inhibitors for the development of eEF2K-targeted therapeutics.


 Please wait while we load your content...
Please wait while we load your content...