De novo design of type II topoisomerase inhibitors as potential antimicrobial agents targeting a novel binding region†
Abstract
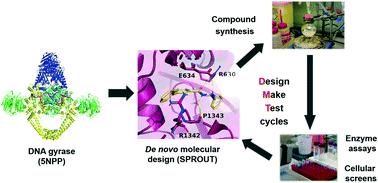
By 2050, it is predicted that antimicrobial resistance will be responsible for 10 million global deaths annually, more deaths than cancer, costing the world economy $100 trillion. Clearly, strategies to address this problem are essential as bacterial evolution is rendering our current antibiotics ineffective. The discovery of an allosteric binding site on the established antibacterial target DNA gyrase offers a new medicinal chemistry strategy. As this site is distinct from the fluoroquinolone binding site, resistance is not yet documented. Using in silico molecular design methods, we have designed and synthesised a novel series of biphenyl-based inhibitors inspired by a published thiophene-based allosteric inhibitor. This series was evaluated in vitro against Escherichia coli DNA gyrase and E. coli topoisomerase IV with the most potent compounds exhibiting IC50 values towards the low micromolar range for DNA gyrase and only ∼2-fold less active against topoisomerase IV. The structure–activity relationships reported herein suggest insights to further exploit this allosteric site, offering a pathway to overcome developing fluoroquinolone resistance.


 Please wait while we load your content...
Please wait while we load your content...