N-1,2,3-Triazole–isatin derivatives: anti-proliferation effects and target identification in solid tumour cell lines†
Abstract
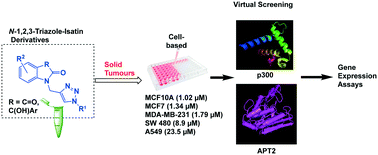
Molecular hybridization approaches have become an important strategy in medicinal chemistry, and to this end, we have developed a series of novel N-1,2,3-triazole–isatin hybrids that are promising as tumour anti-proliferative agents. Our isatin hybrids presented high cytotoxic activity against colon cancer cell line SW480, lung adenocarcinoma cell line A549, as well as breast cancer cell lines MCF7 and MDA-MB-231. All tested compounds demonstrated better anti-proliferation (to 1-order of magnitude) than the cis-platin (CDDP) benchmark. In order to explore potential biological targets for these compounds, we used information from previous screenings and identified as putative targets the histone acetyltransferase P-300 (EP300) and the acyl-protein thioesterase 2 (LYPLA2), both known to be involved in epigenetic regulation. Advantageous pharmacological properties were predicted for these compounds such as good total surface area of binding to aromatic and hydrophobic units in the enzyme active site. In addition, we found down-regulation of LYPLA2 and EP300 in both the MCF7 and MDA-MB-231 breast cancer cells treated with our inhibitors, but no significant effect was detected in normal breast cells MCF10A. We also observed upregulation of EP300 mRNA expression in the MCF10A cell line for some of these compounds and the same effect for LYPLA2 mRNA in MCF7 for one of our compounds. These results suggest an effect at the transcriptional regulation level and associated with oncological contexts.

 Please wait while we load your content...
Please wait while we load your content...