Greatly enhanced electrochemical nitrate-to-ammonia conversion over an Fe-doped TiO2 nanoribbon array†
Abstract
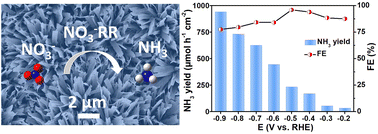
Electrochemical nitrate (NO3−) reduction is an appealing approach to remove NO3− contaminants and simultaneously convert them into high value-added ammonia (NH3), but it requires highly active catalysts with high selectivity. Herein, Fe doping is proposed as an effective strategy to greatly enhance the electrocatalytic NO3−-to-NH3 conversion over a TiO2 nanoribbon array supported on a Ti plate. Such an Fe–TiO2/TP catalyst achieves a large NH3 yield of up to 940.17 μmol h−1 cm−2 and a high NH3 faradaic efficiency of 95.93% under alkaline conditions, superior to those of its undoped TiO2/TP counterpart (57.47 μmol cm−2 h−1, 35.77%). Moreover, it also shows excellent electrochemical and structural stability. Theoretical calculations reveal that Fe doping can dramatically improve the electronic conductivity of TiO2 and optimize the adsorption of reactive species on its surface.


 Please wait while we load your content...
Please wait while we load your content...