Novel methane activation by sulfur dioxide and molecular oxygen via trifluoroacetylsulfuric acid†
Abstract
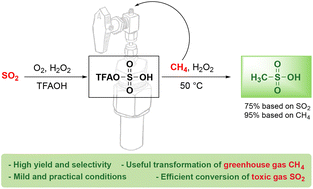
Sulfur trioxide and sulfuric acid have been used for the conversion of methane (CH4) to methanesulfonic acid (MSA), whereas their precursor sulfur dioxide (SO2) has rarely been used. Herein, we report a novel methane sulfonation method using SO2 and its newly explored free radical mechanism. We developed practical conditions to transform SO2 to trifluoroacetyl sulfuric acid (TFAOSO3H) by reacting with molecular oxygen in trifluoroacetic acid. At 50 °C, the resulting TFAOSO3H facilitates the carbon–hydrogen bond activation of methane to efficiently generate MSA via a radical mechanism with hydrogen peroxide as the radical initiator. As limiting reagents, SO2 and CH4 were selectively converted to MSA in 74 and 95% yields, respectively. Since the greenhouse gas CH4 and the toxic gas SO2 can be fully used to produce MSA as a value-added transportable liquid product, this pragmatic one-pot two-step protocol would have great significance in environmental and economical applications.


 Please wait while we load your content...
Please wait while we load your content...