Nitrogen-doped nickel-molybdenum oxide as a highly efficient electrocatalyst for benzyl alcohol oxidation†
Abstract
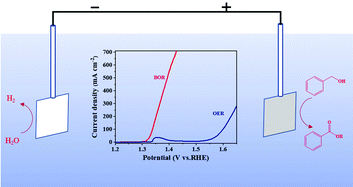
Replacing sluggish oxygen evolution reaction (OER) with electrocatalytic alcohol oxidation to construct hybrid water electrolysis systems is more economically attractive than conventional water splitting. Meanwhile, the electrooxidation of benzyl alcohol is regarded as a green alternative for the conventional synthesis of benzoic acid under moderate conditions. Here, we report the nitrogen-doped nickel-molybdenum oxide (N–Mo–Ni/NF) loaded on Ni foam through a one-step hydrothermal method for the selective electrooxidation of benzyl alcohol to benzoic acid in alkaline electrolytes. The N–Mo–Ni/NF electrode shows intriguing electrocatalytic activity and stability at the anode and only requires a low potential of 1.338 V (vs. RHE) to achieve a current density of 100 mA cm−2 in a 0.1 M benzyl alcohol solution, which is 252 mV lower than that of water oxidation. In addition, a yield of 98.2% of benzoic acid has been obtained with high faradaic efficiency of 98.7%. This work provides a facile strategy to design advanced non-precious electrocatalysts for the oxidation of benzyl alcohol.


 Please wait while we load your content...
Please wait while we load your content...