Mechanochemical Fischer indolisation: an eco-friendly design for a timeless reaction†
Abstract
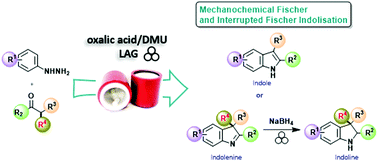
We developed an environmentally friendly mechanochemical protocol to induce an effective Fischer indolisation to synthesize indoles and indolines taking advantage of oxalic acid and dimethylurea. The solvent-free procedure shows versatility and wide scope; it applies to a broad range of arylhydrazines and carbonyl compounds, leading to variously decorated indoles and indolenines. Upon suitable modification, the methodology can also allow preparing compounds of pharmaceutical interest.


 Please wait while we load your content...
Please wait while we load your content...