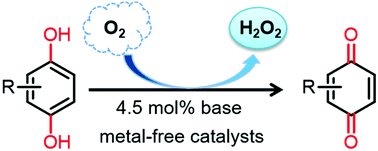
The base-catalyzed aerobic oxidation of hydroquinones to benzoquinones under metal-free conditions†
Abstract
Metal catalysts have always been used in the aerobic oxidation of hydroquinones to benzoquinones. Here an organic base was used as a catalyst to carry out this reaction in the presence of molecular oxygen, which produces hydrogen peroxide (H2O2) as the by-product under metal-free conditions. Various bases, reaction times, temperatures, and solvents were examined, along with the scope of the reaction. The relationship between the dissociation constant (pKa) values of different organic bases and the conversion of hydroquinones was studied. The effects of water on this reaction were also observed. It is shown that the role of the base in the reaction process is to promote proton transfer from hydroquinones, which is exacerbated by the protic nature of the solvent. The results of mechanistic studies suggested that this reaction probably occurs via an ionic pathway. This simple base-catalyzed aerobic oxidation provides a green alternative for the manufacture of benzoquinones from hydroquinones.


 Please wait while we load your content...
Please wait while we load your content...