Inherently chiral helicene-substituted thioalkyl porphyrazine complexes: synthesis and electronic and chiroptical properties†
Abstract
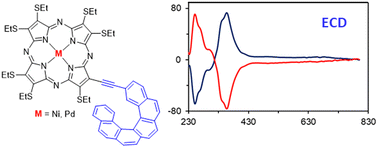
The chiral Ni(II) and Pd(II) complexes of [6]helicene thioethyl porphyrazine have been synthesized and their spectroscopic, electrochemical, and chiroptical properties have been investigated by experimental and computational analyses. In these compounds, the tetrapyrrole macrocycle is β-substituted with an inherently chiral extended aromatic moiety potentially suitable to establish attractive π–π interactions with nanocarbons and endowed with helical chirality, both features providing interesting properties for optoelectronic applications. Experimental and density functional theory computational analyses highlight the presence of HOMO–LUMO charge-transfer transitions between the helicene moiety and the porphyrazine macrocycle. These compounds behave as mono-substituted push–pull systems without any typical electron-withdrawing or electron-donating groups and thus appear promising for optoelectronics. The enantiomers of the Ni(II) complex have been separated by chiral HPLC and their absolute configuration has been established by density functional theory computational analysis of electronic circular dichroism spectra. The magnetic circular dichroism spectrum of this complex has also been recorded providing better insight into its electronic structure.


 Please wait while we load your content...
Please wait while we load your content...