Abstract
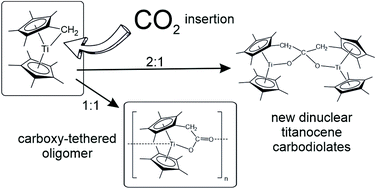
Both single tucked-in permethyltitanocene 1 and double tucked-in permethyltitanocene 2 react with excess CO2 by insertion into their Ti–CH2 bonds. The former one precipitates instantly a yellow carboxylate-tethered oligomer [3]n which is insoluble in aprotic solvents and in a vacuum it sublimes as a monomer without decomposition. Computations for n ≤ 4 optimised the structure of the monomer [3] and showed that open chain oligomers bound by dative O → Ti bonds were not sterically hindered. The latter bond dissociates when [3]n is oxidized by chlorination with CDCl3 or CD2Cl2 to give Ti(IV) chloride 4 or upon metathesis of [3]n with Me3SiCl yielding Ti(III) chloride 5. Oxidative addition of MeCN affords a C–C coupled dinuclear titanocene diimine 6. Compound [3]n also reacts with 1 to give the tethered carbodiolate 8 or with [Cp*2TiH] (where Cp* = η5-C5Me5) to give the half-tethered carbodiolate 10. The non-tethered carbodiolate 12 was obtained from [Cp*2TiH] and CO2 yielding titanocene formate by reaction of the latter with another equivalent of [Cp*2TiH]. All these carbodiolates contain Ti(III) metal atoms forming electronic triplet states of axial or orthorhombic symmetry. In contrast to the rapidly reacting 1 compound 2 reacts with excess CO2 slowly in m-xylene at 100 °C using only one of its two Ti–CH2 moieties. The structure of the obtained carbodiolate 13 indicates that the primary product analogous to 3 reacts with 2 more rapidly than with CO2.


 Please wait while we load your content...
Please wait while we load your content...