Norbornene based-sulfide-stabilized silylium ions: synthesis, structure and application in catalysis†
Abstract
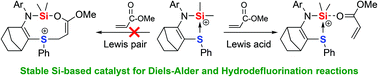
A norbornene-based sulfide stabilized silylium ion 4 has been synthesized. The S–Si interaction was studied in solution and in the solid state by NMR spectroscopy and X-ray diffraction analysis as well as DFT calculations. Unlike the previously reported phosphine-stabilized silylium ion VII, behaving as a Lewis pair, calculations predict that 4 should behave as a Lewis acid toward acrylate derivatives. Indeed, the base-stabilized silylium ion 4 has emerged as an easy-to-handle silylium ion-based Lewis acid catalyst, particularly for the Diels–Alder cycloaddition, with poorly reactive dienes, and hydrodefluorination reactions.


 Please wait while we load your content...
Please wait while we load your content...