Investigations of the reactivity, stability and biological activity of halido (NHC)gold(i) complexes†
Abstract
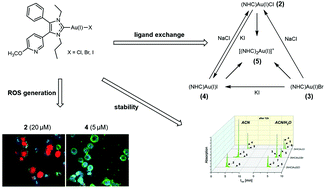
The significance of the halido ligand (Cl−, Br−, I−) in halido[3-ethyl-4-phenyl-5-(2-methoxypyridin-5-yl)-1-propyl-1,3-dihydro-2H-imidazol-2-ylidene]gold(I) complexes (2–4) in terms of ligand exchange reactions, including the ligand scrambling to the bis[3-ethyl-4-phenyl-5-(2-methoxypyridin-5-yl)-1-propyl-1,3-dihydro-2H-imidazol-2-ylidene]gold(I) complex (5), was evaluated by HPLC in acetonitrile/water = 50:50 (v/v) mixtures. In the presence of 0.9% NaCl, the bromido (NHC)gold(I) complex 3 was immediately transformed into the chlorido (NHC)gold(I) complex 2. The iodido (NHC)gold(I) complex 4 converted under the same conditions during 0.5 h of incubation by 52.83% to 2 and by 8.77% to 5. This proportion remained nearly constant for 72 h. The halido (NHC)gold(I) complexes also reacted very rapidly with 1 eq. of model nucleophiles, e.g., iodide or selenocysteine (Sec). For instance, Sec transformed 3 in the proportion 73.03% to the (NHC)Au(I)Sec complex during 5 min of incubation. This high reactivity against this amino acid, present in the active site of the thioredoxin reductase (TrxR), correlates with the complete inhibition of the isolated TrxR enzyme at 1 μM. Interestingly, in cellular systems (A2780cis cells), even at a 5-fold higher concentration, no increased ROS levels were detected. The concentration required for ROS generation was about 20 μM. Superficially considered, the antiproliferative and antimetabolic activities of the halido (NHC)Au(I) complexes correlate with the reactivity of the Au(I)–X bond (2 < 3 < 4). However, it is very likely that degradation products formed during the incubation in cell culture medium participated in the biological activity. In particular, the high-cytotoxic [(NHC)2Au(I)]+ complex (5) distorts the results.


 Please wait while we load your content...
Please wait while we load your content...