Synergistic effect of the MoO2/CeO2 S-scheme heterojunction on carbon rods for enhanced photocatalytic hydrogen evolution†
Abstract
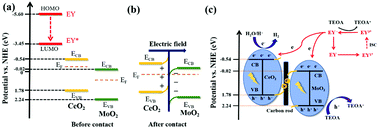
The separation efficiency of photogenerated carriers is a key factor affecting photocatalytic hydrogen evolution activity. However, loading precious metals is a cost problem, so in this work cheap carbon rods are introduced into the S-scheme heterojunction of CeO2/MoO2 as electron transfer channels. The construction of the S-scheme heterojunction greatly improves the reduction activity of a single catalyst and effectively inhibits the recombination of photogenerated electrons and holes. The carbon rods at the interface between CeO2 and MoO2 can ensure the rapid transfer of space charge, thus significantly improving the separation efficiency of photogenerated carriers. The synergistic effect of these two promotes the composite catalyst's photocatalytic hydrogen evolution activity. After optimization, the photocatalytic hydrogen evolution amount of 30% CeO2/MoO2–C (6725 μmol g−1) is 18.6 and 2.43 times those of CeO2 (373 μmol g−1) and MoO2–C (2771 μmol g−1), respectively. 30% CeO2/MoO2–C showed good stability in the photocatalytic cycle experiment. Simultaneously, steady-state fluorescence and electrochemical characterization showed that the introduction of carbon rods promoted the spatial transfer of electrons. This work provides a new design idea and method for applying and developing the S-scheme heterojunction.


 Please wait while we load your content...
Please wait while we load your content...