Synergistic effect of Cu/Cu2O surfaces and interfaces for boosting electrosynthesis of ethylene from CO2 in a Zn–CO2 battery†
Abstract
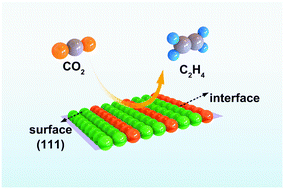
Metal–carbon dioxide batteries have received considerable research interest because the technology not only can be used as clean energy storage devices, but also can achieve the conversion of carbon dioxide to produce useful fuels and chemicals. However, the development of high-performance electrocatalysts remains a major challenge. Here, we report the design and controllable construction of efficient Cu/Cu2O electrocatalysts with synergy of active surfaces and interfaces for the conversion of carbon dioxide to ethylene. The highly active Cu/Cu2O electrocatalysts are relatively stable under the condition of CO2 electroreduction, and the synergistic effect of the Cu/Cu2O surface and interface improves the selectivity and efficiency for C2H4 production. A high ethylene faradaic efficiency of 50% at −1.1 V vs. RHE on the Cu/Cu2O catalyst is achieved. Moreover, Zn–CO2 batteries equipped with the Cu/Cu2O catalyst exhibit an energy density of up to 1.17 mW cm−2, and can maintain a cycle stability of 24 hours. Theoretical calculations show that the surface/interface of catalysts can enhance the adsorption of *CO, and further promotes the dimerization of *CO to ethylene. In situ ATR-FTIR spectroscopy studies have also further confirmed that *CO is an important intermediate during the formation of C2H4. This research will provide a new strategy for improving the stability of C–C coupling products and CO2 capture, conversion and electric power generation.


 Please wait while we load your content...
Please wait while we load your content...