The mechanism of direct reductive amination of aldehyde and amine with formic acid catalyzed by boron trifluoride complexes: insights from a DFT study†
Abstract
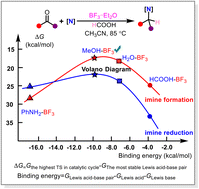
Efficient synthesis of amines using green and economical organic chemical strategy is of importance in chemistry-related industries. Direct reductive amination (DRA) is one of the most convenient and preferred procedures for amine synthesis. Herein we used the density functional theory (DFT) method to investigate the reaction mechanism of DRA of aldehyde and amine catalyzed by boron trifluoride (BF3) complexes with formic acid as the reducing agent. The possible BF3-related active catalytic species in the DRA reaction were discussed. This work pointed out that BF3–H2O species could be the catalytic species with the best catalytic activity in this reaction and that the rate-determining step is the hydride transfer from a formate anion to protonated imine. Based on the constructed volcano diagram of catalytic activities and stabilities of possible BF3-related catalytic species, BF3–MeOH is proposed to have a better catalytic effect than BF3–H2O in reductive amination.


 Please wait while we load your content...
Please wait while we load your content...