Chemoenzymatic deracemization of lisofylline catalyzed by a (laccase/TEMPO)-alcohol dehydrogenase system†
Abstract
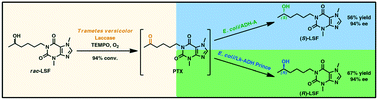
Lisofylline (LSF) is a synthetic methylxanthine active agent exhibiting potent anti-inflammatory and immunomodulatory properties; therefore, it has been widely investigated as a promising drug candidate for treating various autoimmune disorders, including type 1 diabetes. In this study, we report on developing a sequential chemoenzymatic one-pot two-step deracemization protocol for racemic LSF. This task was accomplished in a stereo-complementary manner via a tandem bi-enzymatic oxidation–reduction reaction sequence composed of (i) non-selective chemoenzymatic aerobic oxidation of LSF to pentoxifylline (PTX) catalyzed by commercially available laccase from Trametes versicolor (LTv) and 2,2,6,6-tetramethylpiperidinyloxy radical (TEMPO) as a redox mediator, and (ii) stereoselective bioreduction of in situ formed PTX to give enantiomeric LSF, which was catalyzed by home-made lyophilized E. coli cells harboring overexpressed alcohol dehydrogenases (ADHs) with complementary stereospecificity. Firstly, a multi-step optimization procedure of LTv/TEMPO-catalyzed oxidation of LSF allowed achieving dramatic improvement of the conversion rates from an initial 16% up to 95%, demonstrating the high synthetic potency of this method compared to traditional chemical reactions requiring toxic oxidants used in stoichiometric amounts. In turn, separate stereoselective bioreductions of PTX using recombinant ADHs from Rhodococcus ruber (E. coli/ADH-A) and Lactobacillus kefiri (E. coli/LK-ADH Prince) furnished both LSF enantiomers (>99% ee) with high 93–94% conversion and in 65–67% yield range, respectively. The coupling of the above-mentioned chemoenzymatic steps afforded both antipodes of LSF on a preparative scale (0.16 mmol of racemic LSF) in the range of 56–67% yield and 94% ee depending on the employed ADHs.
- This article is part of the themed collection: Biocatalysis: A cross-journal collection


 Please wait while we load your content...
Please wait while we load your content...