Structure determination and bonding properties of gas-phase OPt2− anion and its neutral form†
Abstract
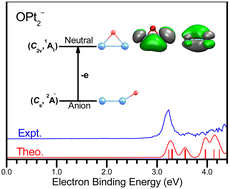
Using anion photoelectron spectroscopy and quantum chemical calculations, the structures and properties of OPt2−/0 are investigated. The vertical detachment energies (VDEs) of OPt2− are measured to be 3.28 eV and 3.23 eV through the use of 355 and 266 nm photons, respectively. The high experimental VDEs of OPt2− can be attributed to the strong Pt–Pt and Pt–O σ bonds and low orbital energy of the SOMO. It is found that neutral OPt2 has an OPt2 triangular structure with C2v symmetry and 1A1 electronic state. In the neutral OPt2, the O atom interacts with the Pt2 moiety by two 2c–2e PtO bonds, one 3c–2e Pt2O σ bond, and one 3c–2e Pt2O π bond. On the other hand, anionic OPt2 adopts a Pt–Pt–O bent structure with Cs symmetry and 2A′ electronic state. NPA and ELF analyses indicate charge transfer upon complexation from the metal atoms to the O atom. Chemical bonding analyses show that OPt2−/0 have the strong covalent Pt–Pt and Pt–O bonds, and neutral OPt2 exhibits σ aromaticity and π antiaromaticity.


 Please wait while we load your content...
Please wait while we load your content...