ALS-associated A315E and A315pT variants exhibit distinct mechanisms in inducing irreversible aggregation of TDP-43312–317 peptides†
Abstract
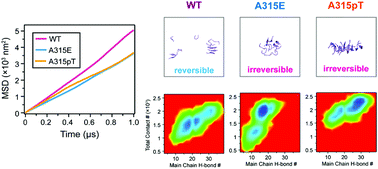
Amyotrophic lateral sclerosis (ALS) is intensively associated with insoluble aggregates formed by transactivation response element DNA-binding protein 43 (TDP-43) in the cytoplasm of neuron cells. A recent experimental study reported that two ALS-linked familial variants, A315E and A315pT (pT, phosphorylated threonine), can induce irreversible aggregation of the TDP-43 312NFGAFS317 segment (TDP-43312–317). However, the underlying molecular mechanism remains largely elusive. Here, we investigated the early aggregation process of the wild type (WT) 312NFGAFS317 segment and its A315E and A315pT variants by performing multiple microsecond all-atom molecular dynamics simulations. Our simulations show that the two variants display lower fluidity than WT, consistent with their decreased labilities observed in previous denaturation assay experiments. Despite each of the two variants carrying one negative charge, unexpectedly, we find that both A315E mutation and A315pT phosphorylation enhance intermolecular interactions and result in the formation of more compact oligomers. Compared to WT, A315E oligomers possess low β-sheet content but a compact hydrophobic core, while A315pT oligomers have high β-sheet content and large β-sheets. Side chain hydrogen-bonding and hydrophobic interactions as well as N312–E315 salt bridges contribute most to the increased aggregation propensity of the A315E mutant. By contrast, main chain and side chain hydrogen-bonding interactions, side chain hydrophobic and aromatic interactions, are crucial to the enhanced aggregation capability of the A315pT variant. These results indicate that glutamate mutation and phosphorylation at position 315 induce the irreversible aggregation of TDP-43312–317 peptides through differential mechanisms, which remind us that we should be careful in the investigation of the phosphorylation effect on protein aggregation by using phosphomimetic substitutions. This study provides mechanistic insights into the A315E/A315pT-induced irreversible aggregation of TDP-43312–317, which may be helpful for the in-depth understanding of ALS-mutation/phosphorylation-associated liquid-to-solid phase transition of TDP-43 protein aggregates.


 Please wait while we load your content...
Please wait while we load your content...