Oxidation of HOSO˙ by O2 (3Σg−): a key reaction deciding the fate of HOSO˙ in the atmosphere†
Abstract
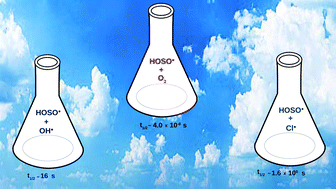
In the present work, we have studied the oxidation of HOSO˙ by O2 (3Σg−) employing quantum chemical and kinetic calculations. The present work reveals that HOSO˙ + O2 (3Σg−) is a barrierless reaction which proceeds through a stable hydrogen-bonded complex. The estimated atmospheric lifetime of HOSO˙ in the presence of O2 (3Σg−) is found to be several orders of magnitude less compared to the other oxidation paths of HOSO˙, suggesting that the oxidation of HOSO˙ by O2 (3Σg−) might be the most dominant oxidation path of HOSO˙ in the atmosphere.


 Please wait while we load your content...
Please wait while we load your content...