Low pressure metastable single-bonded solid nitrogen phases†
Abstract
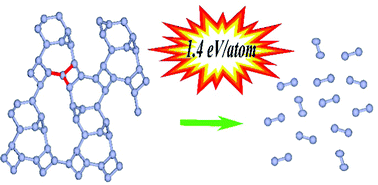
Within the framework of the density functional theory, the possibility of the formation of single-bonded solid atomic nitrogen phases as a result of adiabatic compression of molecular and cluster nitrogen structures at zero temperature has been studied. It has been demonstrated that nitrogen clusters N8(C2v)–B, which are theoretically predicted as one of the promising candidates for high energy density materials, can transform under compression into a solid atomic phase with crystal lattice symmetry P21. The P21 phase is dynamically stable under decompression to zero pressure. It is shown that the ε-N2 molecular phase transforms under compression into a solid atomic phase with R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c symmetry, and retains a vibrationally stable crystal structure when the pressure is reduced to 30 GPa, transforming into a stable cluster form at lower pressures. The atoms in the P21 and R
c symmetry, and retains a vibrationally stable crystal structure when the pressure is reduced to 30 GPa, transforming into a stable cluster form at lower pressures. The atoms in the P21 and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c solid atomic phases are linked by single bonds; therefore, these structures can store a large amount of energy ≈1.4 eV per atom. A detailed comparison of the properties of new P21 and R
c solid atomic phases are linked by single bonds; therefore, these structures can store a large amount of energy ≈1.4 eV per atom. A detailed comparison of the properties of new P21 and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c solid atomic phases with other nitrogen crystal structures that are dynamically stable at low pressures has been carried out.
c solid atomic phases with other nitrogen crystal structures that are dynamically stable at low pressures has been carried out.


 Please wait while we load your content...
Please wait while we load your content...