Contrasting ground- and excited-state intramolecular aggregation in choline chloride-based deep eutectic solvents versus a liquid polymer†
Abstract
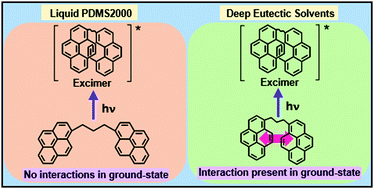
Although the pyrenyl (Py) groups in several dipyrenyl (or bispyrenyl) compounds do not dimerize in the ground-state, they are known to intramolecularly aggregate exclusively in the excited-state to form excimers in common organic solvents. We present contrasting intramolecular aggregation behaviour of such a prototypical compound, 1,3-bis(1-pyrenyl)propane [1Py(3)1Py], dissolved in judiciously selected liquids having relatively high dynamic viscosities (η). Specifically, the intramolecular aggregation of 1Py(3)1Py is investigated in a liquid polymer polydimethylsiloxane with number average MW 2000, PDMS2000 (η293.15K = 21.4 mPa s), and is compared with aggregation of 1Py(3)1Py in deep eutectic solvents (DESs) constituted of the H-bond acceptor (HBA) choline chloride (ChCl) and H-bond donors (HBDs) urea and glycerol in a 1 : 2 mole ratio of ChCl : urea (η293.15K = 1372.0 mPa s) and ChCl : Gly (η293.15K = 473.0 mPa s), respectively, in 293.15 to 363.15 K temperature range. The HBD constituent of ChCl : Gly, glycerol (Gly) (η293.15K = 1412.0 mPa s) is also investigated for comparison purposes. It is found that while in PDMS2000, 1Py(3)1Py intramolecularly aggregates exclusively in the excited-state, thus forming a classical excimer, ground-state heterogeneity is clearly evident in both the DESs and Gly. High viscosity, a consequence of the extensive H-bonding in DESs/Gly, appears to induce the two Py units, both in the ground-state, to exist partly in a configuration where the interaction, albeit a weak one, takes place between the two. This ground-state interaction is not present in relatively low viscosity media PDMS2000 as observed in the common organic solvents. The role of viscosity/H-bonding of the solubilizing milieu on differential intramolecular aggregation in ground- and excited-states is highlighted.


 Please wait while we load your content...
Please wait while we load your content...