Proton binding to humic nano particles: electrostatic interaction and the condensation approximation†
Abstract
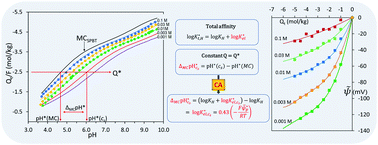
Proton binding to “carboxylic” and “phenolic” sites of humic nano particles (HNPs) is determined by the total proton affinity that is due to a specific and an electrostatic affinity. Both affinities are accounted for in the bi-modal Langmuir–Freundlich (bi-LF)-equation extended with a Boltzmann factor that includes the electrostatic site potential(s), y. For y → 0 the equation reduces to the bi-LF Master Curve (MC). Commonly, an electrical double layer model is used to obtain y, e.g., the bi-LF-Donnan-Vapp (monocomponent NICA-Donnan) model and bi-LF-soft-particle-Poison–Boltzmann-Theory (SPBT). A new method is presented that combines the “condensation approximation” (CA) with the MC concept (CA-MC). With the CA, the proton binding curve and MC can be transformed in, respectively, the total and specific affinity distribution. The difference at a given charge density provides the electrostatic affinity and CA-potentials vs. charge density. The MC can be obtained theoretically or by using the convention that the electrostatic interaction is negligible at 1 M salt concentration. For five HNPs CA-potentials corresponding with the bi-LF-SPBT are compared with results of the bi-LF-Donnan-Vapp model using the MC(SPBT). The bi-LF-Donnan-Vapp model fails when the Debye length > hydrated particle radius. The CA-MC(1M) method does not require characteristics of the HNPs. Combination of the bi-LF-eq. with the CA-MC(1 M) method gives the bi-LF-CA-MC(1 M) model. The CA-MC(1 M) differs from the MC(SPBT); therefore, resulting parameters can only be compared when the same method is used.


 Please wait while we load your content...
Please wait while we load your content...