The lowest-energy structure of the gold cluster Au10: planar vs. nonplanar?†
Abstract
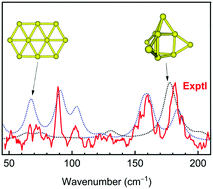
The onset of the transition from 2D to 3D structures in pure gold clusters remains a matter of continuing debate. In this theoretical study we revisit several planar and non-planar structural motifs of the size Au10 with the aim to clarify this issue. Computations using a long-range corrected exchange–correlation functional LC-BLYP, coupled-cluster theories CCSD(T) and PNO-LCCSD(T)-F12 reveal that, at variance with previous reports on the preference of a planar elongated hexagon, both planar and nonplanar isomers of the neutral Au10 are energetically degenerated up to 300 K. Its 3D equilibrium geometry is a core–shell structure which can be built up from a trigonal prism by capping four extra Au atoms outside. A comparison to the available experimental vibrational spectra allows us to argue that both lowest-lying 2D and 3D isomers of Au10 likely coexist in the molecular beam during measurement of its FIR spectra. This result also suggests that the 2D–3D transition of neutral gold clusters occurs at the size Au10.


 Please wait while we load your content...
Please wait while we load your content...