Photodissociation of the trichloromethyl radical: photofragment imaging and femtosecond photoelectron spectroscopy†
Abstract
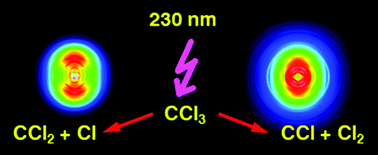
Halogen-containing radicals play a key role in catalytic reactions leading to stratospheric ozone destruction, thus their photochemistry is of considerable interest. Here we investigate the photodissociation dynamics of the trichloromethyl radical, CCl3 after excitation in the ultraviolet. While the primary processes directly after light absorption are followed by femtosecond-time resolved photoionisation and photoelectron spectroscopy, the reaction products are monitored by photofragment imaging using nanosecond-lasers. The dominant reaction is loss of a Cl atom, associated with a CCl2 fragment. However, the detection of Cl atoms is of limited value, because in the pyrolysis CCl2 is formed as a side product, which in turn dissociates to CCl + Cl. We therefore additionally monitored the molecular fragments CCl2 and CCl by photoionisation at 118.2 nm and disentangled the contributions from various processes. A comparison of the CCl images with control experiments on CCl2 suggest that the dissociation to CCl + Cl2 contributes to the photochemistry of CCl3.


 Please wait while we load your content...
Please wait while we load your content...