Plumbing the uncertainties of solvothermal synthesis involving uranyl ion carboxylate complexes†
Abstract
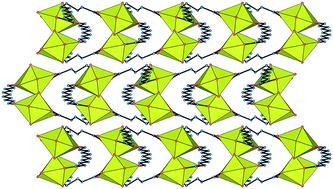
Some of the uncertainties inherent to solvo-hydrothermal synthetic methods which often hinder isolation of the desired product are discussed and illustrated by the structural characterisation of four uranyl ion complexes with long-chain saturated or unsaturated aliphatic dicarboxylate ligands, this being placed in the context of previous results. [Zn(phen)2(HCOO)][UO2(muc)(HCOO)] (1), where H2muc is trans,trans-muconic (trans,trans-1,6-hexa-2,4-dienedioic) acid, includes formate anions, generated in situ from N,N-dimethylformamide (DMF) hydrolysis, as chelating ligands on both metal centres, which limits polymer periodicity. [UO2(muc)(NMP)] (2) was obtained in the presence of PPh4+ cations, but coordination of N-methyl-2-pyrrolidone (NMP) results in formation of a neutral monoperiodic polymer instead of an anionic one. Similarly, NMP complexation prevents inclusion of [Co(en)3]3+ cations in [UO2(C8)(NMP)] (3), where H2C8 is 1,8-octanedioic acid, another monoperiodic coordination polymer. No solvent is coordinated in [H2NMe2]2[(UO2)2(C13)3] (4), where H2C13 is 1,13-tridecanedioic acid, but the desired [Co(en)3]3+ counterions are displaced by H2NMe22+ cations generated in situ from DMF hydrolysis, giving a diperiodic network with the KIa topological type, isomorphous to that formed from 1,15-pentadecanedioic acid. Complexes 1 and 2 are non-emissive in the solid state, while 4 displays a broad uranyl emission peak with unresolved fine structure.


 Please wait while we load your content...
Please wait while we load your content...