Portrayal of the color polymorphism in the 5-acetyl-derivative of ROY†
Abstract
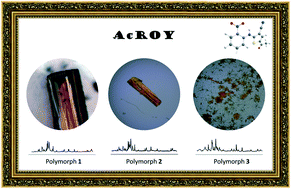
A novel derivative of the prominent ROY compound, 5-acetyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile (AcROY), was synthesized in a two-step procedure by the nucleophilic aromatic substitution reaction between 1-fluoro-2-nitrobenzene and 2-aminothiophene-3-carbonitrile, followed by Friedel–Crafts acylation at position 5 of the thiophene ring. The conformational space of the compound (isolated molecule) was studied computationally, rendering 4 low-energy intramolecularly hydrogen-bonded conformers. The compound exhibits color polymorphism, with 3 different polymorphs identified. The crystal structures of two of the polymorphs were solved by single-crystal X-ray diffraction crystallography: polymorph 1 (burgundy) is monoclinic (P21/n; a = 5.3354(2) Å, b = 14.2344(4) Å, c = 17.1709(6) Å, β = 96.567(2)°, Z = 4, Z′ = 1), and polymorph 2 (orange) is monoclinic (P21/c; a = 25.451(3) Å, b = 14.4966(14) Å, c = 7.1113(7) Å, β = 95.795(6)°, Z = 8, Z′ = 2). It was not possible to determine the crystal structure of the third polymorph (3, orange-yellowish), but the obtained powder X-ray diffraction and infrared and Raman spectroscopy data clearly demonstrate the existence of this additional polymorphic form. Indexing of its powder diffraction pattern showed that it is a monoclinic variety with lattice parameters a = 11.383(3) Å, b = 13.6609(3) Å, c = 25.247(7) Å, and β = 101.75(1), thus exhibiting a more complex supramolecular structure compared to polymorphs 1 and 2. All polymorphs were characterized spectroscopically (by infrared and Raman spectroscopy) and by thermal analysis (by differential scanning calorimetry and polarized light thermomicroscopy). Interestingly, among the 4 predicted low energy conformers of the AcROY molecule, only the most stable form was found to be present in the crystals of the two structurally characterized polymorphs of the compound. The dominant intermolecular interactions in polymorphs 1 and 2 were investigated (also using Hirshfeld surface analysis) and were found to be significantly different. The observed polymorphism of AcROY is then an interesting case of packing-determined color polymorphism.


 Please wait while we load your content...
Please wait while we load your content...