Electro-oxidation induced O–S cross-coupling of quinoxalinones with sodium sulfinates for synthesizing 2-sulfonyloxylated quinoxalines†
Abstract
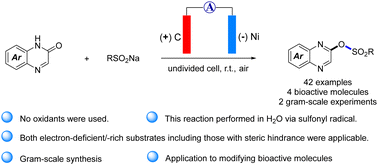
The functionalization of quinoxalinones is synthetically and biologically appealing, however, C2 functionalized quinoxalinones is not reported via environmentally friendly approach. Herein, we disclosed C2–O sulfonylation of quinoxalinones via our developed electrochemical oxidative O–S coupling strategy for synthesizing 2-sulfonyloxylated quinoxalines. Applying this protocol, quinoxalin-ones and sodium sulfinates as the starting materials, a wide range of 2-sulfonyloxyl quinoxaline derivatives were obtained in moderate to good yields with good functional-group tolerance under mild conditions without additional oxidants. The utility of this methodology and the sulfonyloxyl handles was demonstrated trough gram-scale preparation and the synthesis of 2-substituted quinoxaline-based bioactive molecules, respectively.


 Please wait while we load your content...
Please wait while we load your content...