A diversity oriented clicking strategy: the stereoselective synthesis of highly-functionalised olefins from 2-substituted-alkynyl-1-sulfonyl fluorides†
Abstract
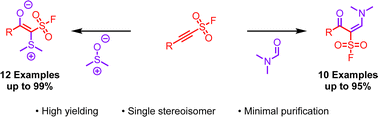
A novel series of addition reactions of highly reactive 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) hubs with DMSO and DMF for the synthesis of two unique sulfonyl fluoride cores is described. The stereoselective chemistry allowed the unprecedented syntheses of 12 (Z)-2-(dimethylsulfonio)-2-(fluorosulfonyl)-1-substitutedethen-1-olates and 10 (E)-1-(dimethylamino)-3-oxo-3-substitutedprop-1-ene-2-sulfonyl fluorides from DMSO and DMF, respectively. The reactions proceed expediently to give single products in excellent yield without the need for chromatographic purification. Furthermore, the utility of the DMSO derived products is demonstrated in the synthesis of synthetically valuable β-keto sulfonyl fluorides under hydrogenation conditions in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...