Visible-light-promoted iron-catalyzed C–H functionalization of 1,4-naphthoquinones via oxidative coupling with sulfoximines†
Abstract
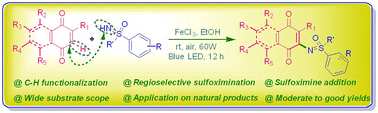
A catalytic oxidative addition of sulfoximines to naphthoquinones via C–H functionalization has been achieved using an iron catalytic system, which exhibits good reactivity and high regioselectivity in the presence of visible light. This is the first report offering an efficient protocol for obtaining (naphtho)quinone-sulfoximine hybrid analogs in moderate to good yields with wide scope for both the substrates. This protocol has also been applied on natural products for their modification, including vitamin K3, Juglone and some other modified natural scaffolds as well.


 Please wait while we load your content...
Please wait while we load your content...